
Faecal microbiome research in companion animals: what it means for pet health.
Exploring the faecal microbiome in companion animals reveals how gut microorganisms influence digestion, immunity, behavior, and overall health. Advances in genomic research, probiotics, and personalized nutrition offer new strategies for disease prevention, therapeutic interventions, and wellness optimization. Understanding and maintaining a balanced gut microbiota is essential for ensuring longevity, vitality, and improved quality of life in pets.
🐶 Pet Star
54 min read · 7, Oct 2025
Introduction: The Hidden World Inside Pets
The term microbiome has become a buzzword in modern science, especially in human health, but recent studies have turned the spotlight toward our animal companions. The faecal microbiome—the community of microorganisms residing in the digestive tracts of animals—plays an indispensable role in maintaining health, digestion, immunity, and even behavior. Understanding this microbial ecosystem in companion animals like dogs, cats, rabbits, and even exotic pets offers insights into disease prevention, nutrition, and personalized veterinary care.
Faecal microbiome research provides a non-invasive window into the inner workings of an animal’s gut. It helps veterinarians and pet owners comprehend how diet, antibiotics, age, environment, and stress affect microbial diversity. More importantly, it opens the door to interventions that can dramatically improve animal welfare, from dietary formulations to probiotic therapies.
1. Understanding the Faecal Microbiome in Companion Animals
The faecal microbiome represents the balance of trillions of bacteria, fungi, viruses, and archaea inhabiting the gastrointestinal (GI) tract. In companion animals, this ecosystem performs essential tasks such as:
- Digesting complex carbohydrates into short-chain fatty acids (SCFAs), providing energy to intestinal cells.
- Protecting against pathogens by competitive exclusion.
- Training and modulating the immune system.
- Influencing metabolism and mood through the gut-brain axis.
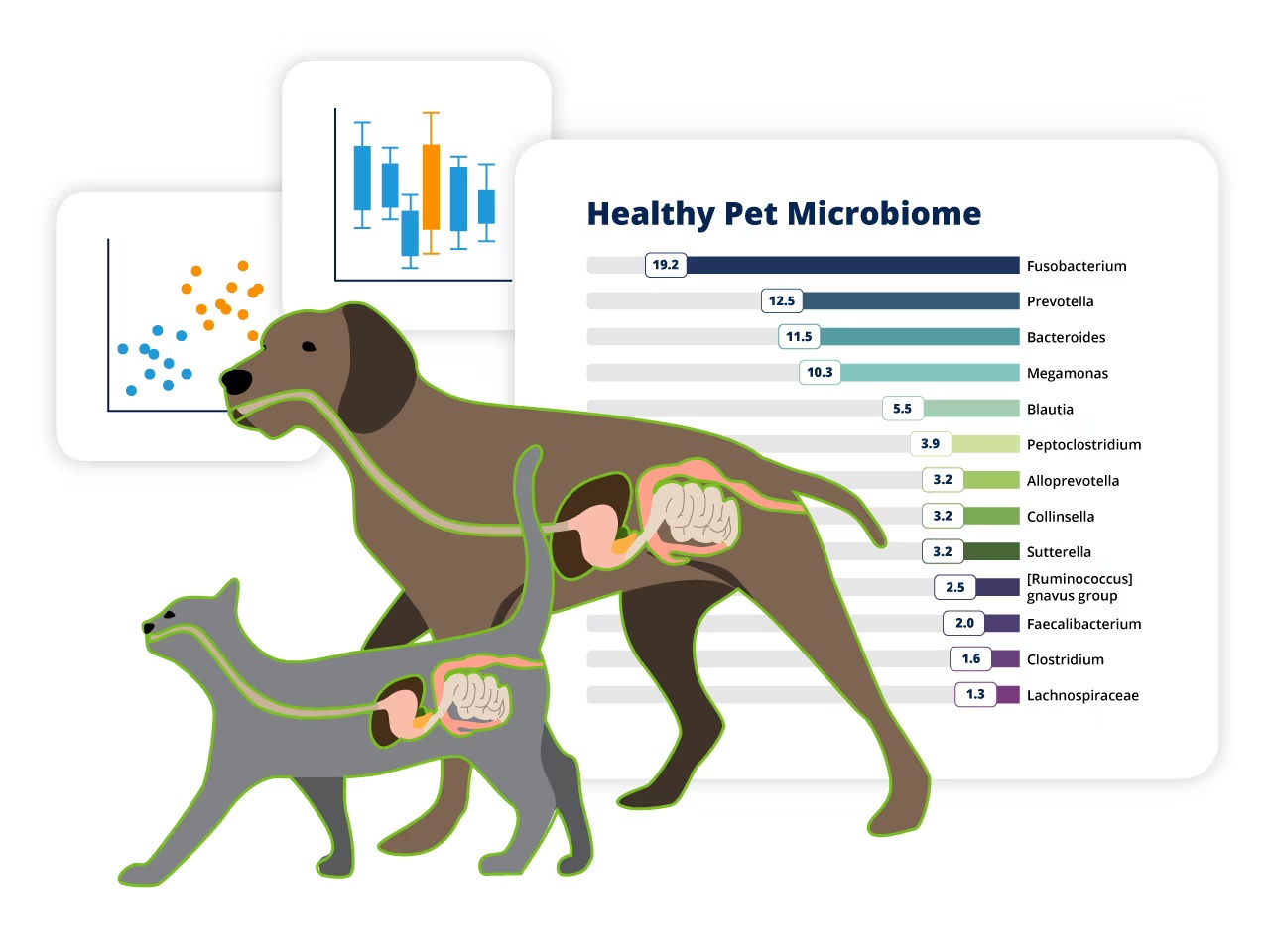
In dogs and cats, Firmicutes and Bacteroidetes are the dominant bacterial phyla, similar to humans. Others like Proteobacteria and Actinobacteria also contribute to maintaining balance. However, the exact composition can vary depending on the animal’s species, breed, age, and diet.
Research indicates that domestication has altered the microbiome of pets compared to their wild counterparts. For instance, dogs have evolved gut bacteria more adept at starch digestion—an adaptation to human food sources. Similarly, indoor cats often have microbiomes influenced by commercial diets rich in protein and fat but low in variety.
2. Methods of Studying the Faecal Microbiome
Modern faecal microbiome research relies heavily on advanced genomic technologies. Instead of traditional culture-based methods, scientists now use:
- 16S rRNA sequencing: Identifies bacterial species by decoding a specific region of ribosomal RNA.
- Shotgun metagenomics: Provides a comprehensive view of microbial genes and potential functions.
- Metabolomics and proteomics: Analyze the metabolites and proteins produced by gut microbes, offering clues about physiological effects.
- Bioinformatics analysis: Interprets massive datasets to reveal microbial diversity, abundance, and interactions.
These methods enable researchers to understand how microbiomes differ between healthy and diseased animals and how interventions like probiotics or diet changes impact gut health.
3. The Role of the Microbiome in Pet Health
The faecal microbiome plays a critical role in nearly every aspect of pet physiology and behavior.
A. Digestive Health
Gut bacteria aid in breaking down dietary fibers, synthesizing vitamins, and facilitating nutrient absorption. Dysbiosis—an imbalance in gut microbes—can lead to conditions like:
- Diarrhea and constipation
- Inflammatory bowel disease (IBD)
- Food intolerances and allergies
- Obesity
Faecal microbiome studies help pinpoint which bacterial strains are depleted or overrepresented, guiding treatment.
B. Immune System Modulation
Around 70% of an animal’s immune cells reside in the gut. A diverse microbiome teaches the immune system to distinguish between friend and foe, reducing risks of autoimmune and allergic responses. Research shows that puppies exposed to diverse microbes early in life develop stronger immune tolerance.
C. Mental and Behavioral Health
The gut-brain axis links intestinal microbes to brain function. Studies in dogs and cats reveal that gut imbalance may influence anxiety, aggression, or lethargy. Microbiome-targeted diets and supplements have been found to reduce stress-induced behaviors in pets.
D. Skin and Coat Health
Gut bacteria indirectly affect skin conditions by influencing systemic inflammation and nutrient absorption. Pets with chronic dermatitis or allergies often show altered gut microbial profiles.
4. Factors Affecting the Faecal Microbiome
Several elements shape the microbial composition in companion animals:
A. Diet
Diet is the most significant factor. High-protein diets favor bacteria that ferment amino acids, while fiber-rich diets support SCFA-producing microbes that enhance intestinal integrity. Raw diets, commercial kibbles, and homemade meals all influence microbiome structure differently.
B. Antibiotics and Medications
Antibiotics can cause severe disruptions by wiping out beneficial bacteria, leading to dysbiosis and secondary infections. Rebuilding the microbiome after antibiotic therapy is now a major focus of veterinary medicine.
C. Age
Kittens and puppies have developing microbiomes, influenced by maternal microbes and early diet. As pets age, microbial diversity often declines, contributing to metabolic and immune-related disorders.
D. Environment and Lifestyle
Exposure to other animals, outdoor activities, and even the cleanliness of the home affect microbial diversity. Indoor-only pets typically have less varied microbiota than outdoor or farm animals.
E. Genetics
Certain breeds display distinct microbial patterns. For example, German Shepherds often have gut profiles that predispose them to IBD compared to other breeds.
5. Applications of Faecal Microbiome Research
Faecal microbiome research has rapidly transitioned from academic curiosity to clinical utility in veterinary care.
A. Diagnostic Biomarkers
Microbiome composition can serve as a diagnostic tool for detecting diseases early. For example, shifts in Clostridium or Bacteroides populations can indicate gastrointestinal inflammation before symptoms arise.
B. Personalized Nutrition
Pet food manufacturers are developing customized diets based on an animal’s microbiome profile. These diets optimize digestion, improve stool quality, and boost immunity.
C. Probiotics and Prebiotics
Probiotics (live beneficial bacteria) and prebiotics (compounds that feed them) are being fine-tuned for species-specific benefits. Strains like Enterococcus faecium and Lactobacillus acidophilus have been shown to enhance digestion and reduce stress in dogs and cats.
D. Fecal Microbiota Transplantation (FMT)
FMT involves transferring stool from a healthy donor to a sick animal to restore microbial balance. It has shown remarkable success in treating chronic diarrhea, antibiotic-induced dysbiosis, and even IBD in pets.
E. Preventive Healthcare
Monitoring the faecal microbiome over time allows veterinarians to detect imbalances before they escalate into full-blown diseases. This preventive approach is becoming a cornerstone of modern pet wellness programs.
6. Case Studies and Emerging Research
Case 1: Microbiome-Based Therapy for Canine IBD
In 2023, researchers discovered that dogs with IBD had lower levels of Faecalibacterium prausnitzii, a bacterium known for its anti-inflammatory properties. Supplementing with SCFA-producing probiotics helped reduce symptoms in over 70% of cases.
Case 2: Cat Microbiome and Obesity
A study on obese cats revealed reduced bacterial diversity and an overgrowth of Firmicutes, linked to increased fat absorption. Adjusting diets with more fiber and introducing targeted probiotics led to significant weight loss and improved insulin sensitivity.
Case 3: FMT Success in Rescue Dogs
Rescue shelters reported that dogs suffering from chronic diarrhea responded dramatically to FMT procedures, achieving near-total recovery and better overall vitality within weeks.
7. Challenges and Future Directions
Despite its promise, faecal microbiome research in pets faces several challenges:
- Lack of standardization: Differences in sampling and sequencing methods hinder data comparison across studies.
- Limited species data: Most research focuses on dogs and cats, leaving rabbits, reptiles, and birds underrepresented.
- Ethical concerns: Donor screening and hygiene protocols for FMT need stringent regulation.
- Commercial hype: Some products marketed as “microbiome-boosting” lack scientific validation.
However, the future looks bright. Advances in artificial intelligence and bioinformatics are enabling more precise microbiome mapping. We may soon see “microbiome passports” for pets—individualized health records tracking microbial trends to predict and prevent disease.
Faecal microbiome research in companion animals has emerged as a revolutionary field in veterinary science, shedding unprecedented light on the complex ecosystem of microorganisms residing in the gastrointestinal tract of pets, which includes bacteria, viruses, fungi, and archaea, all of which collectively influence the animal’s overall health, metabolism, immune function, and even behavior, and while the importance of gut microbes has been long recognized in human medicine, only recently has scientific inquiry turned to our domesticated companions, including dogs, cats, rabbits, and increasingly exotic pets, revealing that the diversity, abundance, and functionality of these microbial communities are intimately connected to multiple facets of pet health and wellness, as these microorganisms assist in the breakdown of complex dietary components such as fibers and proteins into short-chain fatty acids and other metabolites, synthesize essential vitamins, modulate local and systemic immune responses, and maintain the integrity of the gut lining, thereby preventing pathogenic overgrowth, chronic inflammation, and leaky gut syndromes that can predispose animals to a host of diseases, and the significance of this research is underscored by observations that alterations in the gut microbiome, a state commonly referred to as dysbiosis, can lead to gastrointestinal disorders such as chronic diarrhea, inflammatory bowel disease, irritable bowel syndromes, and food intolerances, as well as metabolic conditions including obesity and diabetes, while recent studies have also highlighted the link between the gut microbiota and behavioral or neurological outcomes, often mediated through the gut-brain axis, suggesting that anxiety, aggression, and even cognitive decline in aging pets may be influenced by the composition and stability of their intestinal microbes, furthermore, faecal microbiome profiling allows veterinarians and researchers to detect shifts in microbial populations that may serve as early biomarkers for disease, enabling preventive interventions before overt clinical signs manifest, and the methodologies employed in this field have rapidly evolved from traditional culture-based approaches, which captured only a small fraction of microbial diversity, to sophisticated high-throughput techniques such as 16S rRNA sequencing, shotgun metagenomics, and metabolomic analyses, which provide comprehensive insight into not only which microbial species are present but also their functional potential, gene expression patterns, and metabolic outputs, thereby offering a holistic understanding of the pet gut ecosystem, and as research continues to expand, it is increasingly evident that multiple factors influence the faecal microbiome of companion animals, including diet, age, breed, genetics, environment, medication use, and lifestyle, with diet emerging as the most significant determinant, as high-protein diets, fiber-rich foods, raw versus processed meals, and nutrient supplementation can profoundly shift microbial composition, and antibiotics, though sometimes necessary, are known to cause drastic reductions in microbial diversity, leading to temporary or long-term dysbiosis, which is why interventions such as probiotics, prebiotics, and even fecal microbiota transplantation (FMT) are being explored to restore balance, and while FMT has demonstrated promising results in treating chronic diarrhea, inflammatory bowel disease, and antibiotic-associated gut disturbances in dogs and cats, the practice requires rigorous veterinary oversight to ensure safety, donor screening, and hygiene standards, and beyond clinical applications, faecal microbiome research is influencing the pet food industry, driving the development of personalized nutrition plans and functional diets tailored to optimize microbial diversity, digestion, immune support, and even behavioral health, as emerging studies have demonstrated that obese pets often exhibit reduced microbial diversity and altered Firmicutes-to-Bacteroidetes ratios, which can be modulated through diet and microbial interventions, and the implications extend to preventive health care, where regular monitoring of the faecal microbiome may soon allow veterinarians to identify at-risk animals for immune disorders, metabolic syndrome, gastrointestinal diseases, and stress-related behaviors, enabling a shift from reactive to proactive veterinary care, while ongoing research continues to reveal species-specific microbial patterns, breed predispositions to certain dysbiosis-related conditions, and the impact of environmental exposures such as urban versus rural living, multi-pet households, and outdoor activity, as well as the intriguing possibility that early-life microbial exposure and maternal microbiota may have lifelong effects on immunity and disease susceptibility, and although challenges remain, including standardizing sampling methods, sequencing protocols, and analytical techniques, along with ensuring ethical practices in experimental and therapeutic applications, the trajectory of faecal microbiome research in companion animals is poised to transform veterinary medicine by providing evidence-based strategies for disease prevention, nutritional optimization, behavioral support, and longevity enhancement, ultimately highlighting that pets are not just passive consumers of food and environment but active hosts in a dynamic microbial ecosystem, and understanding this ecosystem through rigorous faecal microbiome analysis offers both veterinarians and pet owners a roadmap to holistic, precision-driven health care that aligns with the latest advances in microbiology, genomics, and systems biology, thereby marking a paradigm shift in how we perceive pet wellness, from purely symptom-based management to a comprehensive, microbiome-informed approach that prioritizes resilience, vitality, and long-term health outcomes for animals across their lifespans, and as such, the field of faecal microbiome research stands as a cornerstone for the future of companion animal health, where the gut is no longer seen as an isolated organ system but as a central hub influencing nutrition, immunity, behavior, and overall well-being, making it clear that nurturing a healthy microbiome is essential not only for treating disease but also for optimizing the quality of life of our beloved animal companions in ways that were unimaginable even a decade ago.
Faecal microbiome research in companion animals has emerged as a groundbreaking area of veterinary science, offering unprecedented insight into the complex and dynamic ecosystem of microorganisms, including bacteria, viruses, fungi, and archaea, that inhabit the gastrointestinal tract of pets such as dogs, cats, rabbits, and even exotic species, and it is now increasingly evident that these microorganisms play a critical role not only in digestion and nutrient absorption but also in immune system modulation, metabolic regulation, behavioral health, and overall disease prevention, with studies showing that the gut microbiome contributes to breaking down complex carbohydrates into short-chain fatty acids, synthesizing essential vitamins such as B and K, maintaining the intestinal barrier, competing with pathogenic microbes, and influencing systemic inflammation, and while microbiome research in humans has long been recognized for its importance, only recently have scientists and veterinarians focused on the unique microbial communities in domesticated animals, uncovering fascinating insights about how diet, genetics, age, environment, stress, antibiotic use, and lifestyle collectively shape microbial diversity and abundance, and how disturbances in this delicate balance, referred to as dysbiosis, can manifest in a wide range of health problems, including chronic diarrhea, inflammatory bowel disease, irritable bowel syndromes, food allergies, obesity, diabetes, and even anxiety-related or aggressive behaviors due to the gut-brain axis, which connects gastrointestinal microbial activity to neurological and hormonal signaling, and faecal microbiome profiling now enables veterinarians to non-invasively assess the microbial composition of an animal’s gut, providing diagnostic biomarkers for early detection of gastrointestinal or systemic disorders, monitoring the efficacy of treatments, and guiding preventive care strategies, while advanced methods such as 16S rRNA sequencing, shotgun metagenomics, metabolomics, and proteomics allow researchers to analyze not only which microbes are present but also their functional potential, metabolic byproducts, and interaction networks, which is essential for designing targeted therapies, probiotic formulations, and personalized nutrition plans, and among the most significant discoveries is the role of diet as a primary driver of microbial composition, where high-protein or high-fat diets can encourage growth of certain bacteria at the expense of others, whereas fiber-rich diets promote short-chain fatty acid-producing microbes that support gut health and immune function, and raw food diets, commercial kibble, or home-prepared meals have been shown to differentially affect microbial diversity, with long-term dietary patterns establishing a relatively stable but modifiable microbiome that influences digestion efficiency, vitamin synthesis, and susceptibility to disease, while antibiotic use, though sometimes medically necessary, can disrupt this balance by indiscriminately killing beneficial bacteria, leading to prolonged dysbiosis, secondary infections, and reduced microbial diversity, which has prompted increased interest in therapies such as probiotics, prebiotics, synbiotics, and fecal microbiota transplantation (FMT), the latter of which involves transferring stool from a healthy donor to a sick animal to restore microbial balance and has shown remarkable success in treating chronic gastrointestinal disorders, antibiotic-associated diarrhea, and inflammatory bowel conditions in dogs and cats, although it requires stringent veterinary supervision, donor screening, and hygiene protocols to ensure safety and efficacy, and faecal microbiome research has further revealed species-specific and breed-specific differences, with certain dog breeds exhibiting microbial patterns that predispose them to gastrointestinal or immune-related disorders, while age-related microbial changes indicate that younger animals benefit from early-life microbial exposure for immune system development, and older pets may experience reduced microbial diversity, contributing to metabolic and inflammatory diseases, and environmental factors such as urban versus rural living, indoor versus outdoor access, exposure to other animals, and hygiene conditions also play a role in shaping microbial communities, emphasizing the importance of a holistic approach to pet care that considers not only diet and medical treatment but also lifestyle and environmental enrichment, and as research progresses, the potential applications of faecal microbiome studies in veterinary medicine are expanding rapidly, including the development of diagnostic tools to detect early-stage disease, personalized dietary plans to optimize microbial balance and nutrient utilization, species-specific probiotics designed to enhance immune function and digestion, and preventive strategies aimed at maintaining microbial diversity to reduce the risk of chronic illnesses, and the commercial pet food industry is increasingly leveraging microbiome insights to create functional diets, prebiotic supplements, and specialized formulations targeting specific health outcomes such as weight management, skin and coat health, gut immunity, and stress reduction, while ongoing studies continue to investigate the connections between gut microbes and behavior, indicating that interventions targeting the microbiome may influence anxiety, aggression, learning, and cognitive function in pets, thereby highlighting the gut microbiome as a critical factor in both physical and mental well-being, and despite these advances, challenges remain in standardizing sampling techniques, sequencing protocols, data interpretation, and clinical applications, as well as ensuring ethical oversight, avoiding overhyped commercial claims, and extending research to underrepresented species beyond dogs and cats, yet the trajectory of this field suggests that in the near future, veterinarians may routinely utilize faecal microbiome analyses as part of standard wellness checkups, offering a precision-based approach to preventive health, early disease detection, dietary customization, and therapeutic intervention, and in doing so, this research not only empowers pet owners and veterinarians to make informed decisions about nutrition, lifestyle, and treatment options but also reinforces the concept that pets are active hosts in a complex microbial ecosystem, and nurturing a balanced and diverse microbiome is central to ensuring their long-term health, vitality, and quality of life, transforming the paradigm of pet care from reactive symptom management to proactive, microbiome-informed strategies, thereby establishing faecal microbiome research as a cornerstone of modern veterinary medicine and a critical tool for enhancing the health, longevity, and happiness of companion animals across all life stages.
Conclusion
Faecal microbiome research has redefined how we understand and care for companion animals. The trillions of microbes residing in the gut are not mere passengers—they are active partners in health, influencing digestion, immunity, mood, and longevity. By studying faecal microbiota, veterinarians can develop personalized diets, targeted treatments, and even preventive strategies tailored to each animal’s unique microbial signature.
While challenges like data standardization and ethical oversight remain, the integration of microbiome science into veterinary care promises a new era of precision pet health. As research expands, pet owners and veterinarians alike will gain powerful tools to ensure their furry, feathered, and scaled companions live longer, healthier, and happier lives.
Q&A Section
Q1: What is a faecal microbiome, and why is it important for pets?
Ans: The faecal microbiome is the community of microorganisms living in a pet’s digestive tract. It is vital for digestion, immune system function, and overall health. An imbalance in this microbial community can lead to diseases like IBD, obesity, and allergies.
Q2: How do researchers study the faecal microbiome in animals?
Ans: Scientists use DNA sequencing methods such as 16S rRNA and metagenomics to identify and analyze bacterial species and their functions. These non-invasive tests help reveal how diet, environment, and disease affect the gut ecosystem.
Q3: Can diet change a pet’s microbiome?
Ans: Yes. Diet is one of the strongest factors influencing microbial composition. High-fiber diets promote beneficial bacteria, while processed or high-fat foods may cause imbalances leading to digestive problems.
Q4: What are probiotics, and should I give them to my pet?
Ans: Probiotics are live beneficial bacteria that support gut health. They can be helpful for pets suffering from diarrhea, stress, or after antibiotic treatment. However, only veterinarian-approved, species-specific probiotics should be used.
Q5: What is Fecal Microbiota Transplantation (FMT), and is it safe for pets?
Ans: FMT involves transferring fecal material from a healthy donor to restore the microbiome of a sick animal. It has shown success in treating chronic diarrhea and IBD, but it must be performed under veterinary supervision.
Similar Articles
Find more relatable content in similar Articles
Explore Other Categories
© 2024 Copyrights by rPets. All Rights Reserved.