
Actiphage for Johne’s and bTB – Herd Health Center discusses the potential of Actiphage in diagnosing Johne’s disease and bovine tuberculosis.
Actiphage is a groundbreaking diagnostic tool that leverages bacteriophage D29 to detect live mycobacteria, offering a more accurate and rapid method for diagnosing Johne’s disease (MAP) and bovine tuberculosis (M. bovis). By identifying active infections through blood samples, Actiphage enables early detection, targeted control strategies, and faster herd health management, ultimately improving disease control, animal welfare, and public health outcomes.
🐶 Pet Star
64 min read · 14, Apr 2025
Introduction
Johne’s disease (JD) and bovine tuberculosis (bTB) are chronic, debilitating infections in cattle caused by Mycobacterium avium subspecies paratuberculosis (MAP) and the Mycobacterium tuberculosis complex (MTC), respectively. Both diseases are notoriously difficult to diagnose due to their slow progression and the limitations of traditional diagnostic methods. However, recent advancements have introduced innovative solutions. One such breakthrough is the Actiphage method, a rapid and sensitive diagnostic tool developed to detect viable mycobacteria in cattle
Understanding Actiphage Technology
Actiphage utilizes bacteriophage D29, a virus that specifically infects and lyses mycobacterial cells. When introduced to a blood sample, D29 targets and infects viable mycobacteria, causing them to release their DNA. This DNA is then extracted and analyzed using polymerase chain reaction (PCR) to identify the presence of MAP or MTC. The method's sensitivity allows for the detection of as few as 10 mycobacterial cells per milliliter of blood, providing a significant advantage over traditional culture-based techniques that can take weeks to yield results.
Application in Diagnosing Johne’s Disease
Johne’s disease is a chronic infection that primarily affects the intestines of cattle, leading to weight loss, diarrhea, and eventually death. Traditional diagnostic methods, such as fecal culture and serological tests, often fail to detect the disease in its early stages, as bacteria may not be shed in detectable amounts. Actiphage addresses this challenge by detecting viable MAP bacteria in the blood, even before clinical symptoms appear
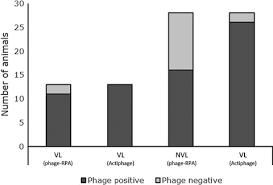
In a study involving experimentally infected cattle, Actiphage identified MAP in the blood of 87% of infected animals, compared to 40% detection by traditional phage-PCR methods. This enhanced sensitivity allows for earlier detection and more effective management of JD in herds.Application in Diagnosing Bovine
Tuberculosis
Bovine tuberculosis, caused by MTC organisms like Mycobacterium bovis, is a significant concern for livestock health and public safety. Conventional diagnostic methods, such as the Single Intradermal Comparative Cervical Tuberculin (SICCT) test, can yield false negatives, particularly in animals with low-level infections.
Actiphage has demonstrated a 95% sensitivity and 100% specificity in detecting MTC in the blood of cattle, even in those with no visible lesions. This high level of accuracy makes Actiphage a valuable tool for confirming bTB infections, especially in cases where traditional tests may be inconclusive.
Advantages of Actiphage Over Traditional Methods
- Rapid Results: Provides results within 6 hours, significantly reducing the time compared to traditional culture methods.
- High Sensitivity: Capable of detecting low levels of viable mycobacteria, improving early detection rates
- No Need for Culture: Eliminates the need for time-consuming and resource-intensive culture procedures
- Viable Cell Detection: Specifically identifies live bacteria, ensuring that the detected DNA is from actively replicating organisms.
- Broad Applicability: Effective across various species, making it suitable for diverse livestock populations.
Implementation in Herd Health Management
Integrating Actiphage® into herd health programs can lead to more effective disease management strategies. Early detection of JD and bTB allows for prompt isolation of infected animals, reducing the risk of transmission to other herd members. Additionally, identifying infections before clinical signs appear can lead to more targeted treatment and control measures, ultimately improving animal welfare and farm productivity.
Summary and Conclusions
Actiphage® represents a significant advancement in the diagnostic capabilities for Johne’s disease and bovine tuberculosis. By enabling the rapid and sensitive detection of viable mycobacteria in blood samples, it addresses the limitations of traditional diagnostic methods. Its high sensitivity and specificity make it an invaluable tool for early disease detection, leading to better-informed management decisions and improved herd health outcomes.
The emergence of Actiphage as a novel diagnostic tool marks a significant advancement in veterinary medicine, particularly in the diagnosis and management of two persistent and economically devastating diseases affecting cattle: Johne’s disease and bovine tuberculosis (bTB). Johne’s disease, caused by Mycobacterium avium subspecies paratuberculosis (MAP), and bovine tuberculosis, caused by Mycobacterium bovis (a member of the Mycobacterium tuberculosis complex), are both chronic infections that pose substantial challenges in terms of detection, control, and eradication. Traditional testing methods, including fecal culture, serological assays, and the tuberculin skin test, have limitations in sensitivity, specificity, and speed, particularly when it comes to identifying subclinical or early-stage infections. These conventional diagnostics often require weeks to yield results and may not accurately detect animals that are infected but not yet shedding the bacteria or showing overt symptoms. This diagnostic gap can lead to delayed or ineffective disease management interventions, allowing infected animals to remain undetected within herds and continue spreading disease silently. Actiphage, developed by PBD Biotech, addresses these limitations by leveraging a powerful biological mechanism—bacteriophage D29, which specifically infects live mycobacterial cells. When introduced into a blood sample, the bacteriophage targets and invades viable MAP or M. bovis cells, causing them to lyse and release their DNA. This DNA is then extracted and amplified using PCR (polymerase chain reaction) to detect the presence of live pathogens with remarkable sensitivity. The most compelling advantage of Actiphage is its ability to detect live infection within hours rather than weeks, offering a transformational shift in how veterinarians and farmers approach disease surveillance and control. For Johne’s disease, where animals may incubate the infection for years before shedding detectable levels of bacteria or exhibiting clinical signs, early detection is crucial. Actiphage makes it possible to identify infected animals during these early, preclinical stages by detecting viable MAP in blood samples, thus enabling informed decisions around culling, isolation, or monitoring to prevent further transmission within the herd. This early diagnostic capability not only helps in controlling the spread of Johne’s disease but also reduces long-term economic losses associated with decreased milk yield, premature culling, and reduced reproductive performance. Similarly, in the context of bovine tuberculosis, the traditional tuberculin skin test and interferon gamma release assays (IGRAs) can sometimes yield false negatives due to immune system suppression, coinfection, or the animal's inability to mount a detectable response. Actiphage circumvents these immunological limitations by directly targeting the pathogen, providing a reliable means to confirm infection in animals that might otherwise go undiagnosed. Studies and pilot programs conducted by veterinary institutions and herd health centers have shown that Actiphage can identify M. bovis in cattle that later display TB lesions upon post-mortem examination, even though they previously tested negative with conventional methods. This suggests that Actiphage could serve as a critical supplementary test in bTB eradication efforts, especially in high-risk herds or regions with endemic infection. The rapid turnaround time—typically within 6 to 8 hours—also allows veterinarians to respond quickly, reducing the window for potential transmission and improving animal welfare by avoiding unnecessary testing or treatment delays. Furthermore, because Actiphage is based on blood sampling, it is significantly less invasive and more practical than fecal sampling, which is often labor-intensive, unpleasant, and inconsistently reliable due to intermittent shedding patterns in infected animals. The convenience of blood testing not only facilitates higher compliance among farmers but also supports more frequent and widespread herd health monitoring. Additionally, Actiphage’s ability to scale for large herds and its compatibility with automation and centralized laboratory systems make it a viable candidate for integration into national surveillance and certification schemes. Its use could be particularly impactful in certifying herds as disease-free with greater confidence, supporting the economic competitiveness of livestock products in domestic and international markets. However, despite its promise, Actiphage is not without challenges. As a relatively new technology, it is still undergoing validation across different breeds, management systems, and geographical regions. Regulatory acceptance varies by country, and its adoption in official testing programs will depend on continued demonstration of its reliability and cost-effectiveness. Moreover, while Actiphage excels in identifying infected animals, decisions regarding the best course of action—such as treatment, culling, or isolation—still depend on broader herd health management strategies and economic considerations. Nonetheless, early adopters in veterinary research, university herd health programs, and progressive farming operations have reported strong confidence in the test's performance, praising its accuracy, speed, and potential to transform mycobacterial disease control. Actiphage is not intended to fully replace traditional diagnostics at this stage but rather to complement them by providing confirmatory evidence in ambiguous cases, enhancing the detection of latent infections, and supporting proactive rather than reactive disease management. Its application also has significant implications for public health, particularly in reducing the zoonotic risk of M. bovis, which can be transmitted to humans through unpasteurized milk or close contact with infected animals. By improving early detection and control in livestock, Actiphage contributes to safer food production systems and a reduction in antimicrobial resistance, as animals can be more accurately diagnosed and managed without resorting to unnecessary or broad-spectrum antibiotic treatments. Looking ahead, researchers are also exploring the broader potential of phage-based diagnostics and their integration with genomic tools for strain typing and epidemiological tracking, which could further enhance our understanding of mycobacterial diseases and how they spread within and between herds. In this light, Actiphage represents not just a test, but a new paradigm in veterinary diagnostics—one that is rooted in molecular biology, precision medicine, and real-time disease management. Its introduction into the field is a timely development, especially as global agriculture faces increasing pressure to ensure food safety, animal welfare, and sustainability. As the technology continues to evolve and gain acceptance, Actiphage could become an indispensable component of herd health strategies worldwide, helping farmers, veterinarians, and policymakers tackle the persistent challenge of Johne’s disease and bTB with greater clarity, efficiency, and confidence.
Actiphage: A Revolutionary Diagnostic Tool for Johne’s Disease and Bovine Tuberculosis
The diagnosis of Johne’s disease (JD) and bovine tuberculosis (bTB) has long been a challenge for veterinary practitioners, particularly because both diseases often remain asymptomatic during early stages of infection. Johne’s disease, caused by Mycobacterium avium subspecies paratuberculosis (MAP), is a chronic bacterial infection that primarily affects the gastrointestinal tract of cattle, leading to severe weight loss, diarrhea, and ultimately death in infected animals. Similarly, bTB, caused by Mycobacterium bovis (part of the Mycobacterium tuberculosis complex), is a zoonotic disease that affects both cattle and humans, causing lesions in the lungs and other organs. Both diseases can result in significant economic losses for farmers due to decreased productivity, increased veterinary costs, and, in some cases, the necessity for culling infected animals. Unfortunately, traditional diagnostic methods for both JD and bTB are not without limitations. In the case of Johne’s disease, traditional methods like fecal culture, PCR-based testing of fecal samples, and serological tests often fail to detect the infection in its early stages, as the bacterium does not always shed detectable amounts of bacteria in the feces or blood until the disease is well advanced. Similarly, the standard diagnostic method for bTB, the tuberculin skin test (TST), can produce false negatives, especially in cattle that are in the early stages of infection or have been exposed to low levels of the pathogen. Furthermore, diagnosing animals that are subclinically infected or carriers of these diseases has always been problematic. This is where Actiphage® comes into play—an innovative diagnostic tool that has the potential to significantly improve the detection of both Johne’s disease and bTB in cattle. Developed by PBD Biotech, Actiphage utilizes bacteriophage D29, a virus that specifically infects and kills mycobacterial cells. When introduced to a blood sample, D29 targets viable MAP or M. bovis bacteria present in the sample, causing them to lyse (break open) and release their DNA into the surrounding fluid. This DNA can then be extracted, amplified, and analyzed using polymerase chain reaction (PCR), allowing for the identification of even minute quantities of living mycobacterial cells. This approach is particularly advantageous because it directly detects live bacteria rather than relying on immune responses or dead bacterial fragments, which is the case with many traditional diagnostic methods. The ability to detect live bacteria makes Actiphage a more reliable indicator of active infection. In the context of Johne’s disease, this breakthrough is especially valuable because it allows for the detection of the disease during its early stages, long before clinical symptoms manifest or before the animal starts shedding bacteria in large enough quantities to be detected using traditional tests like fecal culture. By identifying subclinical carriers of the disease, Actiphage allows farmers and veterinarians to implement targeted control measures, such as culling infected animals or segregating them from healthy animals, which helps prevent the spread of the disease within herds. Additionally, since the test uses blood samples rather than fecal samples, it is non-invasive and can be performed much more quickly and efficiently, providing results in as little as six to eight hours, compared to the weeks required for traditional culture methods. This rapid turnaround time is a significant advantage, particularly in large herds where timely decision-making is critical to minimizing the spread of infection. The potential applications of Actiphage for bovine tuberculosis are equally promising. Traditional bTB testing methods, like the tuberculin skin test, can be slow to identify infected animals, and false negatives can occur, especially in animals that have not yet developed a strong immune response or in low-grade infections. This has made bTB particularly difficult to manage in certain regions, especially in areas where the disease is endemic. Actiphage offers a new diagnostic avenue for bTB by detecting live M. bovis bacteria in the blood, which could help identify infected animals earlier, even before they develop obvious symptoms or lesions. In several trials, Actiphage has been shown to detect bTB in cattle that tested negative on the standard skin test but were later found to have lesions during post-mortem examinations. This suggests that Actiphage could serve as a complementary diagnostic tool for bTB, helping to reduce false negatives and improve the overall accuracy of bTB surveillance programs. Given the zoonotic nature of bTB, which poses a significant risk to human health, improving the accuracy and speed of bTB detection is of paramount importance. Early detection allows for the isolation of infected animals, reducing the risk of transmission to humans and other animals, as well as preventing the unnecessary culling of animals that are not infected. The widespread adoption of Actiphage as part of bTB surveillance programs could significantly enhance the ability of health authorities to control and ultimately eradicate the disease in cattle populations. Furthermore, Actiphage’s non-invasive nature (it requires only blood samples) and its ability to deliver results quickly make it an attractive option for routine herd health monitoring. The ease of use and scalability of Actiphage also makes it suitable for large-scale testing programs, whether on individual farms or as part of national eradication schemes. As the veterinary industry continues to seek more effective, accurate, and timely diagnostic tools, Actiphage presents a new frontier in the management of both Johne’s disease and bTB. Its ability to detect live mycobacteria, coupled with its rapid results and non-invasive sampling method, sets it apart from existing diagnostic technologies. While there are still regulatory hurdles to overcome and further research is needed to validate its use across different geographic regions and cattle breeds, the initial results are promising, and it is expected that Actiphage will play a pivotal role in the future of livestock health management. As part of an integrated disease control strategy, Actiphage could not only help farmers and veterinarians manage these diseases more effectively but also contribute to the broader goal of improving herd health, reducing animal suffering, and minimizing the risk of zoonotic diseases. In conclusion, the development and adoption of Actiphage for the detection of Johne’s disease and bovine tuberculosis could mark a turning point in the way these diseases are diagnosed and controlled. With its ability to detect live infection in both subclinically infected and symptomatic animals, its rapid turnaround time, and its non-invasive nature, Actiphage presents a significant improvement over traditional diagnostic methods. As research continues and more data becomes available, it is likely that Actiphage will become a valuable tool in the ongoing efforts to improve cattle health and reduce the economic and public health impacts of these serious diseases.
Actiphage for Johne’s and bTB – A New Frontier in Bovine Disease Diagnostics
In the evolving world of veterinary diagnostics, Actiphage® has emerged as a highly promising and innovative tool in the battle against two of the most persistent and economically damaging cattle diseases: Johne’s disease (caused by Mycobacterium avium subspecies paratuberculosis, or MAP) and bovine tuberculosis (caused by Mycobacterium bovis, part of the Mycobacterium tuberculosis complex, or MTC). Both conditions are chronic, progressive, and difficult to detect reliably in their early stages, making them a significant challenge for livestock farmers, veterinarians, and animal health authorities. Traditional diagnostic methods for Johne’s disease rely on serology, fecal culture, or PCR on fecal samples, which often detect only late-stage infection when the animal is already shedding bacteria. Similarly, for bTB, the common diagnostic tools—such as the tuberculin skin test (TST) and interferon-gamma assays—can have limitations in sensitivity and specificity, especially in differentiating between exposure and active infection. This is where Actiphage offers a game-changing approach. Actiphage is a blood-based diagnostic test developed by PBD Biotech that employs a bacteriophage (virus that infects bacteria) called D29, which specifically targets and lyses viable mycobacteria. When introduced into a blood sample, the bacteriophage infects any viable MAP or MTC bacteria present and causes them to burst, releasing their DNA. This DNA is then extracted and amplified via polymerase chain reaction (PCR), allowing the detection of even minute numbers of living mycobacterial cells, often before clinical signs appear or before traditional tests yield a positive result. The ability of Actiphage to detect live, active infection sets it apart—it is not simply detecting immune response or dead bacterial fragments but actual, living pathogens. Herd Health Centers and veterinary researchers have highlighted the significance of this for both Johne’s and bTB control programs. For Johne’s disease, early detection is critical for controlling spread within a herd, since MAP has a long incubation period and infected animals can silently spread the pathogen. Actiphage allows for the identification of subclinical animals—those that are infected but not yet symptomatic or shedding—enabling strategic culling or segregation, ultimately reducing environmental contamination and the prevalence of disease in future generations. Furthermore, the test’s non-invasive nature (blood sampling vs. fecal sampling) makes it more acceptable and practical for routine herd screening. When applied to bTB, Actiphage introduces a potentially valuable new layer to existing testing regimes. In trials and field applications, it has demonstrated the capability to detect bTB in animals that tested negative via the skin test but were later found to have lesions at post-mortem. This suggests Actiphage might serve as a supplementary diagnostic tool to reduce false negatives and improve early detection, particularly in high-risk herds or regions. The implications for eradication efforts are profound: faster, more accurate diagnostics could help pinpoint infection sources, identify asymptomatic carriers, and improve disease surveillance and control measures. Another compelling advantage of Actiphage is its speed—results can be obtained within 6–8 hours, compared to the weeks or even months required for traditional culture methods. This rapid turnaround enables timely decision-making, reducing the window during which infected animals might unknowingly spread disease. Herd Health Centers are particularly excited about Actiphage's potential in risk-based testing strategies, targeted screening, and even in certifying disease-free herds with higher confidence. The technology also supports "test and cull" approaches without requiring whole-herd depopulation, which is often economically and emotionally devastating for farmers. Additionally, Actiphage’s compatibility with automation and its scalability makes it suitable for large-scale herd health programs and integration into national disease control frameworks. However, the adoption of Actiphage is not without challenges. Regulatory approvals, cost considerations, and the need for trained personnel to conduct PCR analyses remain hurdles. There is also a need for further validation across different breeds, geographies, and management systems to confirm its robustness and reliability under various field conditions. Nevertheless, early adopters and pilot programs have reported encouraging results. The Herd Health Center at various agricultural universities and livestock research stations have endorsed the inclusion of Actiphage in integrated disease management programs. In particular, they highlight its role in bolstering biosecurity, improving traceability, and supporting evidence-based interventions. From an animal welfare perspective, early diagnosis and removal of infected animals reduce suffering caused by late-stage disease, while improving overall herd health and productivity. From a public health standpoint, particularly concerning bTB, enhanced detection and control reduce the zoonotic risk to humans, especially in regions where pasteurization of milk is inconsistent or where occupational exposure to livestock is common. Furthermore, as antimicrobial resistance becomes a growing concern, the ability to pinpoint infection earlier reduces reliance on broad-spectrum treatments and helps promote more judicious use of antibiotics in veterinary practice. Looking forward, researchers are exploring the expansion of Actiphage’s capabilities to detect other mycobacterial infections in different species, and its potential integration with genomic sequencing for strain typing. Such innovations could open doors to even more personalized and precise disease management protocols. In conclusion, Actiphage represents a significant leap forward in the diagnosis of Johne’s disease and bovine tuberculosis. Its sensitivity, speed, and ability to detect live infection offer new hope in the fight against two of the most difficult-to-manage diseases in cattle. While it may not yet fully replace existing tests, it holds strong potential as a complementary tool that enhances diagnostic confidence, supports early intervention, and contributes to long-term disease control and eradication strategies. Herd Health Centers and veterinarians around the world are closely watching its progress—and in many cases, leading the way in research, adoption, and advocacy for broader use.
Summary and Conclusions
Actiphage represents a significant advancement in the diagnostic capabilities for Johne’s disease and bovine tuberculosis. By enabling the rapid and sensitive detection of viable mycobacteria in blood samples, it addresses the limitations of traditional diagnostic methods. Its high sensitivity and specificity make it an invaluable tool for early disease detection, leading to better-informed management decisions and improved herd health outcomes.
Q&A Section
Q1: What is Actiphage and how does it work?
Ans: Actiphage is a diagnostic method that uses bacteriophage D29 to infect and lyse viable mycobacterial cells in blood samples. The released DNA is then analyzed using PCR to detect the presence of Mycobacterium avium subspecies paratuberculosis (MAP) or Mycobacterium tuberculosis complex (MTC).
Q2: How does Actiphage compare to traditional diagnostic methods?
Ans: Actiphage offers several advantages over traditional methods, including faster results (within 6 hours), higher sensitivity (detecting as few as 10 mycobacterial cells per milliliter), and the ability to detect viable bacteria without the need for culture.
Q3: Can Actiphage detect Johne’s disease in cattle?
Ans: Yes, Actiphage has been shown to detect viable MAP bacteria in the blood of cattle, even before clinical symptoms appear, allowing for earlier diagnosis and intervention.
Q4: Is Actiphage effective for diagnosing bovine tuberculosis?
Ans: Absolutely. Studies have demonstrated that Actiphage can accurately detect MTC in cattle blood, including animals with no visible lesions, providing a reliable method for confirming bTB infections.
Q5: How can Actiphage be integrated into herd health programs?
Ans: Actiphage can be incorporated into routine screening procedures to identify infections early, enabling prompt isolation and treatment of affected animals, thereby reducing the spread of disease within the herd.
Similar Articles
Find more relatable content in similar Articles
Explore Other Categories
© 2024 Copyrights by rPets. All Rights Reserved.